Knowledge of metal depth, methods of improving overall corrosion resistance and strengthening modification of magnesium alloy materials
Release time:2022-02-08Click:985
Removal of impurities to improve corrosion resistance and strengthening improvement. Improving the purity of magnesium alloys generally high purity magnesium alloys than high purity magnesium alloys have better corrosion resistance, improve the purity of magnesium alloys is a commonly used means to improve the corrosion resistance of magnesium alloys. The corrosion rate of magnesium alloys is very low when the impurity content does not exceed their allowable limits. For example, the corrosion resistance of AZ91 and AM60 in salt spray is better than that of A1380(al-4.5 cu-2.5 Si) and cold rolled steel in pressure casting. The improvement of magnesium alloy purity is mainly through metallurgical methods, such as refining magnesium alloy, but the general cost is higher.
2. It is technically difficult and costly to reduce the content of harmful elements in magnesium alloys to a lower level by harmless impurities. In fact, the deleterious removal of impurities does not necessarily mean removal of the impurities from the magnesium alloy. If the impurities can be converted into harmless substances, the corrosion will be fine even if they remain in the alloy. The easy and economical way to achieve this aim is to add some elements which are easy to react with impurities in the magnesium alloy, so that the impurities will combine with these elements and become less harmful to corrosion. The elements that are known to have this function are either manganese or error. Therefore, adding manganese or zirconium in magnesium alloy is the main means of harmless impurity of magnesium alloy. In general, the corrosion resistance of magnesium alloy containing aluminum will be greatly improved by adding some manganese or some zirconium in magnesium alloy without aluminum.
3. ALLOYING is an important means to change the chemical composition, phase composition and microstructure of magnesium alloys. It is an important way to improve the corrosion resistance of magnesium alloys. From the point of view of improving corrosion resistance, the aim of alloying is to promote the corrosion resistance of base phase and the formation and reasonable distribution of corrosion-resistant and barrier phases, so as to improve the corrosion resistance of magnesium alloy. From the view of alloying elements in common use at present, the beneficial element for improving the corrosion resistance of magnesium alloy is aluminum, as long as the magnesium alloy contains the right amount of aluminum, its corrosion resistance is higher than that of the alloy without aluminum. The addition of aluminum, on the one hand, the passivity of the base phase (α phase) is improved, on the other hand, more corrosion resistant β phase is produced, and a continuous network is formed between the grains of α phase, which prevents the corrosion expansion of α phase. But the amount of aluminum should be moderate, otherwise it will affect the other properties of magnesium alloy. In addition, whether β phase has the effect of blocking corrosion, the key is to see whether the formation of an effective barrier network. In addition, rare earth elements and zirconium can also improve the corrosion resistance of magnesium alloys. Rare Earth elements mainly promote the passivity of magnesium alloys, while zirconium may promote the chemical stability of α phase.
Special Casting Technology 1. Die Casting is one of the most commonly used components of magnesium alloys and is particularly suitable for mass production using the mode of production process. When magnesium alloy is cast in cold cavity, the cooling rate of the surface layer is higher, which can be considered as a rapid solidification process, while the cooling rate of the inner part of the casting is lower, which is similar to that of common casting. In this way, the surface layer of rapidly solidified magnesium alloy has certain particularity, now take Az91d die casting for example to explain. Because of the particularity of the casting process, some α phase grains with very low al content were solidified before the molten magnesium alloy was pressed into the die cavity. After being squeezed into the metal pressure die cavity, these solid low AL containing α phase grains tend to be concentrated on the surface of the casting due to the action of the fluid mechanics, while the remaining liquid magnesium alloy has a higher al content. After entering the metal mold cavity, the surface layer is cooled quickly by the metal mold wall because of the high heat capacity of the metal mold. Therefore, there is always a layer of rapidly solidified surface layer on the surface of magnesium alloy die casting parts, the grain is very fine, the quantity of β phase is relatively rich in aluminum, and the distribution is very uniform, and almost continuous β phase network is formed along the intercrystalline. Such a surface layer is obviously beneficial to the corrosion resistance of the die casting. In contrast, in the pressure-cast Magnesium Alloy, the α phase has coarse grain, and the β phase has less and discontinuous distribution, so it is not corrosion-resistant. The corrosion behavior of die cast AZ91D alloy in 1 MOL/L NACL solution with Ph = 11 is shown in Table 1.

In short, because of the cold cavity pressure casting, the surface microstructure of the sample is generally finer than that of the ordinary casting, the distribution of β phase is more continuous, can effectively prevent the development of corrosion, so its corrosion resistance is correspondingly higher. If the process of die casting can be properly optimized, the grain size of the sample surface can be further refined, and the content and continuous distribution of the second phase of corrosion resistance can be increased, the corrosion resistance of magnesium alloy can be further improved.
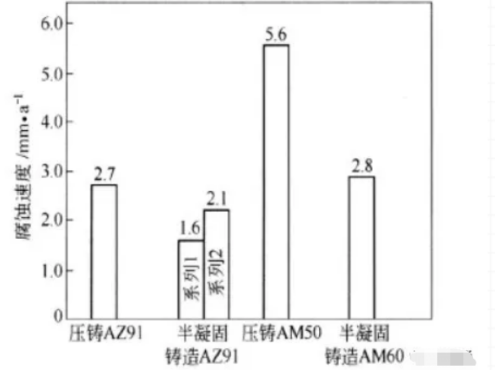
Semi-solidification casting is a new and low-cost casting technology for magnesium alloys. During the semi-solidification casting, the melting temperature of the magnesium alloy is controlled between the liquidus and the solid line, so the magnesium alloy is in the semi-solidification state, about half is solid phase and half is liquid phase. During solidification, the dendrites formed during solidification are broken due to strong agitation. The microstructure of the solid alloy is characterized by large equiaxed because of low casting temperature, low shrinkage, high viscosity, low energy consumption, high yield and long die life. The basic phase grains are surrounded by fine eutectic a phase and other alloy phases, and the content of β phase is a little more. In theory, because these a phase coarse crystals form a continuous quality quality control network, it may effectively prevent the development of α phase corrosion, at the same time, it is possible to make the aluminum content (2.7%) in the coarse-grained phase a higher than that (1.6%) in the common cast magnesium alloy, so the corrosion resistance of the semi-solidified casting is higher. Sometimes the corrosion resistance of semi-solidified magnesium alloys is even slightly higher than that of die casting (see Fig. 1) . Therefore, semi-solidification casting may be one of the means to improve the corrosion resistance of magnesium alloys. But in fact, the corrosion performance of some semi-solidified Az91d is not always better than that of ordinary die casting. It has been found that the corrosion rate of semi-solidified casting is higher at the initial stage in NACL solution, and then decreases at the later stage. It is possible that the cathode and anode of semi-solidified AZ91D alloy are quite different, so the effect of the galvanic cell is large, and the galvanic corrosion is strong at the initial stage. In addition, it has been reported that semi-solidification casting can also improve the oxidation resistance of magnesium alloys under 300 °C.
Strong plastic forming technology such as rolling, forging and extrusion is also an important method to produce magnesium alloy components. Theoretically speaking, these manufacturing processes will change the microstructure of magnesium alloy to a great extent, and therefore the corrosion resistance of magnesium alloy will change. For example, when the amount of deformation is large, rolling, forging and extrusion can change the material from cast structure to processed structure, eliminate the coarse columnar crystal, compress pores, pinholes and porosity, the removal of Dendrites will obviously improve the corrosion resistance, mechanical properties and comprehensive properties of the material. Especially when the alloy composition, deformation temperature and deformation speed are suitable, the new technologies such as superplastic forming or isothermal forging of magnesium alloy and isothermal extrusion can be used to strengthen and modify the structure and properties of the material to a greater extent, of course, its corrosion resistance will also be improved. Equal Channel extrusion (ECAP) is an effective method for grain refinement. The average grain size of AZ31 magnesium alloy can reach 5 μm after four-step equal channel extrusion, while the average grain size of ZK60 magnesium alloy can reach 1.0ー1.4 μm after four-step equal channel extrusion. The comprehensive properties of wrought magnesium alloy can be greatly improved by the combination of equal channel extrusion and proper annealing process.
Solution Heat treatment is an effective method to adjust the phase, composition distribution and grain size of the alloy. It has a great effect on the corrosion of magnesium alloys. The effect of heat treatment on the corrosion resistance of magnesium alloy is obtained by changing the microstructure of magnesium alloy. Taking AZ91E magnesium alloy as an example, the corrosion resistance of both T5 and T6 can be greatly improved, while that of T4 can be greatly reduced. The commonly used heat treatment methods are solution homogenization heat treatment (T4) , solution aging heat treatment (T6) and aging heat treatment (T5) . They can also be used to modify the corrosion resistance of magnesium alloys. According to the results of heat treatment of AZ91 magnesium alloy, the effect of heat treatment on the corrosion resistance of AZ91 magnesium alloy depends on the distribution of the second phase. T4 Heat treatment reduces the alloy precipitate phase and the corrosion rate increases, while T5 and T6 heat treatment cause a lot of β phase to precipitate and form a continuous corrosion barrier layer, so the corrosion rate decreases. Fig. 2 shows the effect of effective heat treatment on the corrosion properties of Az91d die castings. It can be found that the corrosion rate of Az91d die casting reaches the lowest point at about 45 hours of aging. This is related to the change of the composition and distribution of Al and β phases in magnesium alloy during aging heat treatment. As shown in Fig. 3 and Fig. 4, the solid solution aluminum content in the α phase of Az91d die casting decreases with aging time, which is not conducive to the increase of corrosion rate. At the same time, the amount of β phase in the die casting increases with the aging time, and the new β precipitates are mainly distributed in the grain boundary, which is beneficial to resist the development of corrosion. These two opposite corrosion trends change with aging time, resulting in the lowest corrosion rate.
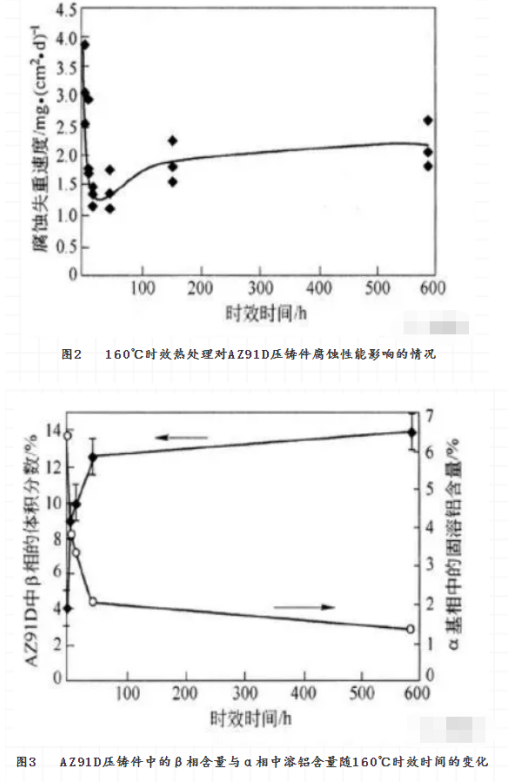
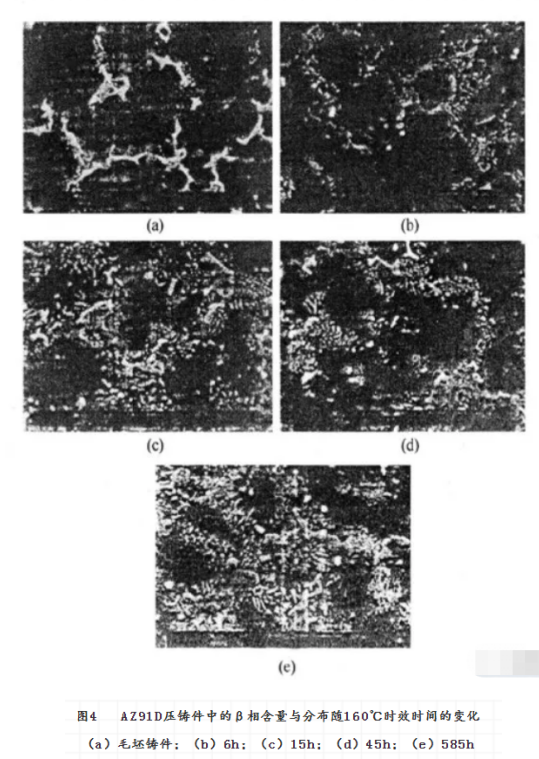
However, for AZ91C with high impurity content, the effect of heat treatment on corrosion performance is not obvious. The corrosion resistance of AZ91 and AM60 alloys is adversely affected by the short time of t 5 aging heat treatment. However, the corrosion resistance of the alloy recovered when aging time was long. This phenomenon can not be explained simply by the change of β phase. The above results show that the corrosion resistance of the magnesium alloy can be improved if the aging heat treatment is well controlled. Rapid solidification is the rapid solidification of Molten Magnesium Alloy, in a protective atmosphere, sprayed to have a high heat capacity of the low-temperature metal mold, so that the rapid cooling of molten magnesium alloy solidification. When the low-temperature metal mould is a runner, a thin magnesium alloy strip with very fine grain can be obtained, and even nanocrystalline or amorphous alloy can be obtained when the composition of magnesium alloy is appropriate The molten magnesium alloy can also be sprayed into a large low temperature metal cavity with high pressure inert gas to obtain a bulk magnesium alloy, rapid solidification of magnesium alloys can also be obtained by rapid cooling of molten magnesium alloys by means of sputtering, vapor deposition and laser treatment.
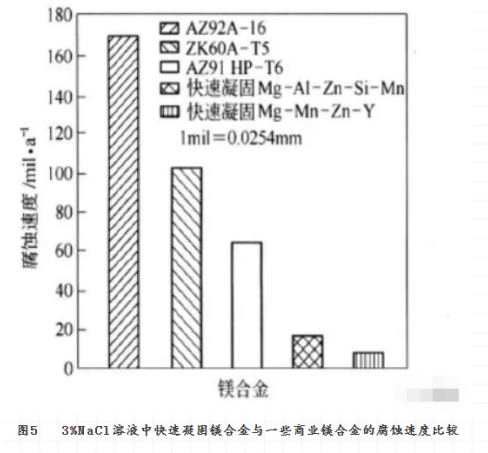
The magnesium alloy made by rapid solidification can not only improve the mechanical properties of the material, but also increase its corrosion resistance. The reasons for improving corrosion resistance are: (1) the formation of new phases may reduce the electrochemical activity of harmful impurities in the new phases, or increase the allowable limit of impurities. (2) the grain size of magnesium alloy is refined or even amorphous, and the phase and composition distribution are homogenized to reduce the activity of micro-galvanic corrosion. (3) increasing the solid solubility of elements beneficial to corrosion resistance in magnesium, thereby reducing the electrochemical activity of magnesium. For example, if there is more solid solution aluminum in magnesium, it is possible to form a surface oxide film with higher aluminum content. In this way, magnesium alloys have better self-passivation and repair, which is beneficial to the corrosion resistance of magnesium alloys. Fig. 5 shows the effect of rapid solidification on the corrosion rate of magnesium alloys.
The composition of magnesium alloy has an important effect on the microstructure of rapidly solidified solid. For example, the crystalline structure of Mg-Ni alloy will weaken with the increase of Ni content, when the NI content is 4.8% (molar fraction) , the phase structure of Mg-Ni alloy will be α-mg and Mg2ni, when the NI content is 18.3% , it will become amorphous completely. Accordingly, the dissolution rate of the alloy in 0.01 MOL/L NACL (PH = 12) solution decreases with the increase of Ni content and the increase of Amorphous degree (see Fig. 6) . The decrease of dissolution rate is related to the increase of passivity of the alloy.
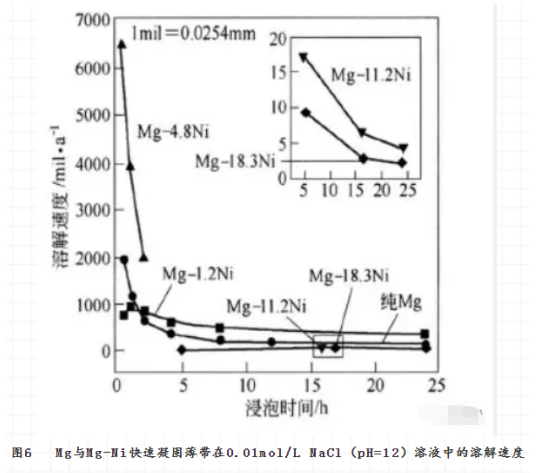
The composition of the alloy also has a critical effect on the corrosion resistance of rapidly solidified magnesium alloys. Fig. 7 shows the effect of different alloy elements on the corrosion rate of rapidly solidified binary magnesium alloys. It can be seen that aluminum is the only alloy element that can improve the corrosion resistance of rapidly solidified binary magnesium alloy. The role of aluminum in rapidly solidified magnesium alloys is to promote passivation and to increase pitting corrosion rupture potential. In the corrosion process, the aluminum is enriched on the surface of the magnesium alloy because of the preferential dissolution of Magnesium, and the protective property of the surface film is enhanced. However, the effect of zinc on corrosivity in rapidly solidified Mg-Zn-Y alloys seems to be less certain. In addition, the corrosion resistance of magnesium can be improved by the solid solution of y element. The solubility of Y in ordinary magnesium is only 3.75% (mole fraction) . In the rapidly solidified magnesium alloy, Y may be higher than this content without precipitation, which is beneficial to the corrosion resistance of the rapidly solidified magnesium alloy. For example, 15% ~ 26% Y (mass fraction) can cause pseudo-passivation zone in rapidly solidified magnesium alloy. Y in rapidly solidified magnesium not only increases the passivity of magnesium, but also increases the pitting rupture potential of magnesium. The addition of y into Mg-Cu alloy not only improves the corrosion resistance of the alloy, but also broadens its passivation zone. This is related to Y2O3 entering into the crystal lattice of Mgo. Rare Earth elements not only increase the heat stability of rapidly solidified alloys, but also benefit the corrosion resistance of rapidly solidified magnesium alloys. It may be related to the following two factors: (1) Rare Earth reacts with solution to form protective film. (2) the second phase in the alloy is passivated and the pitting tendency is reduced. In addition, calcium can also improve the corrosion resistance and thermal stability of rapidly solidified magnesium alloys. Therefore, the addition of calcium and rare earth to rapidly solidified magnesium alloy should be a better choice. Therefore, these effects on the rapid solidification of magnesium alloys can be used to improve the corrosion resistance of magnesium alloys.
1 Amorphous and Amorphous Magnesium alloys not only have higher resistance to local corrosion than crystallized magnesium alloys, but also have higher mechanical properties (strength and toughness) than polycrystalline magnesium alloys. Therefore, great attention has been paid to the development of amorphous magnesium alloys, especially bulk amorphous magnesium alloys. At present, the possibility of forming amorphous magnesium alloys is mainly limited to the following systems: Mg-Zn, Mg-cu, Mg-Ni, Mg-Ca, Mg-TM-Ln, Mg-Y-Ln, Mg-Ca-al, Mg-Zn-al, Mg-Al-Ca. The most promising amorphous magnesium alloy system is Mg-TM-Ln. TM is a transition metal, such as zinc, copper, or nickel; Ln is an element of your family, including y. Y is a key element because Mg-Y has a very high negative enthalpy of mixing. In addition, the atomic volume of lanthanides is much larger than that of magnesium, while copper and nickel are smaller. Therefore, the mixture of the three may have a very high local strain energy, so that when solidified from the molten state, the diffusion rate of the atom should be low and it is difficult to nucleate to form crystal phase. Therefore, Mg-TM-Ln system has excellent amorphous forming ability. In addition, the series of alloys also have extremely high strength. In this series of alloys, Mg65cu25y10 Ternary alloy has the best amorphous forming ability, the cooling rate only needs about 50K/s to make it amorphous. Amorphous alloys such as Mg-TM-Ln (TM = Ni, Cu, Zn) , Mg-Ca-Al, Mg-Al-Y and Mg-Y-Ln have good corrosion resistance. LN In Mg-TM-Ln system is generally beneficial to the corrosion resistance of Amorphous, but TM is disadvantageous to the corrosion resistance of Amorphous. In addition, the effect of other alloying elements on the corrosion resistance of Amorphous magnesium alloys is not the same. The corrosion rate of Amorphous magnesium decreased with the addition of Al, but increased with the addition of Si, CA, Li and Zn.
Nanostructured magnesium alloys can also be produced by microcrystallization and rapid solidification of nanostructured magnesium alloys. The melting mg-12% (mole fraction, same as below) Zn-Ce (3%) and Mg-Zn (10% ~ 20%)-la (0 ~ 10%) MG alloys were sprayed on the low temperature metal wheel to obtain a metal belt of 20 μm thickness. The metal belt is mainly Amorphous, in which the α-mg particles of 3 ~ 20 nm are dispersed, the interparticle distance is about 3 ~ 10nm. High strength nanostructured magnesium alloys can also be obtained by rapidly solidifying Powder metallurgy. The molten magnesium alloy was atomized and solidified rapidly under high pressure argon gas injection to form 25 ΜM fine powder. The powders were cold pressed and then extruded at temperatures higher than the crystallization temperature of the amorphous alloy. The corrosion resistance of magnesium alloy with nanostructure is good. The corrosion resistance of Mg70Ca10Al20 with the nanostructure prepared by the rapid Powder metallurgy method is better than that of Az91d after T6 heat treatment (see Fig. 7) .
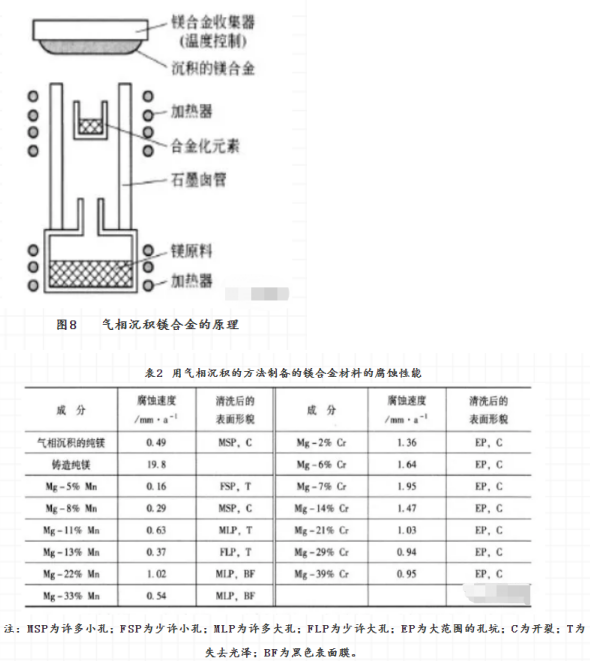
Some binary magnesium alloys, Mg-Zr, Mg-Ti, Mg-V, Mg-Mn and MG-CR, have been successfully prepared by vapor deposition. The corrosion resistance of Mg-Zr, Mg-Ti and Mg-Mn is better than that of pure magnesium, while that of Mg-V and MG-CR is worse. 4 sputtering in the vacuum tube, the Argon molecule is ionized and then bombarded the harrow material containing magnesium under the action of electric field. The atoms from the Harrow material are deposited on the support surface of the sample to form the sputtering magnesium alloy, in the corrosion water of astm d1384, the passivation zone of MgAlZnSn is wider than that of other magnesium alloys, such as Mgzr, MGTA and Mgy. This shows that magnesium alloys with high corrosion resistance can be obtained by this method.
Source: Caiyitong
Disclaimer: Some pictures and texts on this site are collected from the Internet and are only for learning and communication. The copyright belongs to the original author and does not represent the views of our site. This site will not bear any legal responsibility. If your rights are violated, please contact us to delete it in time.


